Explain Different Types of Redox Reactions
It takes the form of X Y XY. All forms of combustion from gasoline in a car engine or gas in our kitchens are redox reactions that involve a compound fuel and oxygen and that release a great deal of energy be it heat light or movement as in the case of explosions.
Redox Reactions Examples Types Applications Balancing
Upon the death of any living organism the organic compounds present in the organism start reacting with oxygen.

. This session will explain about different types of Redox Reaction and how to identify them. Understand the concept of Redox Reaction. Examples of a combustion reaction are fire a combustion engine and fireworks.
Oxidation and reduction are two types of chemical reactions that often work together. Combustion is a type of redox reaction. Different types of Redox Reaction with CBSE Class 11 course curated by Abhay Khedia on Unacademy.
Hence the two types of reactions always redox reaction are Single-displacement reaction and Disproportionation reaction. Combination reaction Decomposition reaction Displacement reaction Double Displacement reaction Precipitation Reaction 1. Examples of naturally occurring precipitation include rain snow and hail.
2Mg O 2 2MgO 2 Decomposition reactions. The reaction in which oxygen is added hydrogen is removed is called oxidation reaction. Combination Reaction Decomposition Reaction Displacement Reaction and Disproportionate Reaction.
Was first used to describe reactions in which metals react with oxygen in air to produce metal oxides. There are different methods of dete point of redox titrntions. All high electronegative elements like N O F Cl etc are oxidants.
The living matter in nature primarily is made up of carbon hydrogen and oxygen. AB AB A B A B. SO 4 aqBaCl 2 aqBaSO 4 s2NaClaq e Oxidation reduction reaction.
Learning Objectives Explain the processes involved in a redox reaction and describe what happens to their various components. Combination Reaction A reaction in which two or more reactants combine to form a single product is known as a combination reaction. Oxidising agents are lewis acid.
All organic reactions have been classified into different types. The Chemistry course is delivered in English. When iron is exposed to air in the presence of water for example the iron turns to.
Substance which can oxidise others and reduce themselves. Is sublimation in organic reaction. Redox reactions are of four types.
In a combination reaction two molecules are combined to form one molecule. Get 1-on-1 help from an expert tutor now. Oxidation and reduction reactions involve an exchange of electrons between reactants.
Answer Write the balanced equation for the following chemical reactions. I Hydrogen Chlorine Hydrogen chloride ii Barium chloride Aluminium sulphate Barium sulphate Aluminium chloride iii Sodium Water Sodium hydroxide Hydrogen 741 Views Answer Why should a magnesium ribbon be cleaned before burning in air. The aforesaid reaction is a prolonged process.
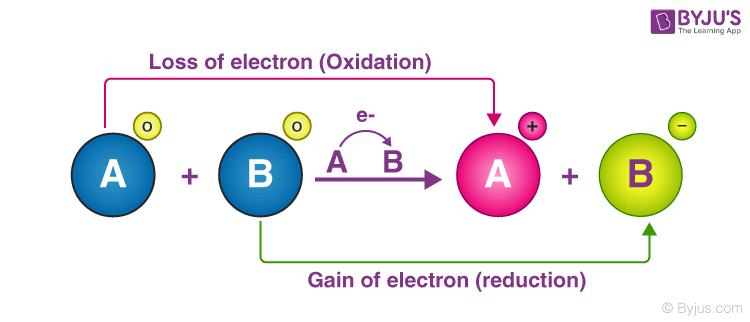
A number of phenomena both physical as well as biological are concerned with redox reactions. In this reaction oxidation takes place when lose electrons and form and reduction takes place when gain electrons and form. The term oxidation refers to t he loss of one or more electrons in a chemical reaction.
Redox reactions are classified into the following types. We have provided some frequently asked questions on types of organic reactions here. Logically oxygen acts as an oxidizing agent removing electrons from the compound.
These reactions find extensive use in pharmaceutical biological industrial metallurgical and agricultural areas. The substance that loses electrons is said to be oxidized. This process commonly referred to as decay or decomposition forms another example of redox reactions.
No sublimation is a method of purifying solids. Usually in a combustion reaction oxygen combines with another compound to form carbon dioxide and water. Key Takeaways Key Points In combination reactions two elements are combined.
Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of atoms are changed. These Notes covered type of redox titration indicator used in redox titratio and application of redox titration with solved Multiple choice questions. Chemical reactions in which a compound splits up into two or more simpler substances are called.
All metallic oxides are oxidants. Example oxidation of copper shown in image The reaction in which hydrogen is added oxygen in removed is called reduction reaction. A combustion reaction is a type of redox reaction in which a combustible material combines with an oxidizer to form oxidized products and generate heat exothermic reaction.
The importance of these reactions is apparent from the fact that burning of different types of fuels for obtaining energy. The five main types of redox reactions are combination decomposition displacement combustion and disproportionation. Reduction recation shown in image.
An example of a combustion reaction is the burning of naphthalene. Precipitation is a reaction that that produces a solid precipitate. For many students the confusion occurs when attempting to identify which reactant was oxidized and which reactant was reduced.
Some non metallic oxides are also oxidants like CO2. Substance which shows decrement in oxidation number. The major types of organic reactions are as follows.
The 5 primary types of chemical reactions are. When two or more substances combine to form a single substance the reactions are called combination reactions. Decomposition reaction involves breaking down one molecule into two or more different molecules.
Substitution Addition elimination and rearrangement reaction. Redox reactions are characterized by the actual or formal transfer of electrons between chemical species most often with one species the reducing agent undergoing oxidation losing electrons while another species the. While a combustion reaction results in heat precipitation occurs due to cooling.

Redox Reactions Examples Types Applications Balancing


No comments for "Explain Different Types of Redox Reactions"
Post a Comment